COURSE DESCRIPTION
Energy can neither be created nor destroyed, but it can be transform into different form. Transformation of energy into usable and more scalable form will be learned in this course. The following topics will be reviewed;
- Heat and work
- Laws of thermodynamics
- Steady flow energy equation
Note: Questions are arranged in descending order
Course Materials
Recommendable, we advise you to download Summaries and Textbooks related to this course. Click on the buttons below
An adiabatic cylinder, sketched below, contains nitrogen gas (modelled as an ideal gas) and a block of copper separated by an adiabatic membrane. Initially (state1), the pressure and the temperature of the gas are 6×10^5Pa and 270K respectively. The copper block temperature is 380K. A heavy adiabatic frictionless piston (M=45kg) is pinned at a height of 20cm above the surface of the copper block. The copper block is 1cm thick. The surface area of the piston is 50cm^2. The density of copper is 8.96g/cm^3. The specific heat capacity of copper is 385 j/kg.K. the gas constant for nitrogen is 297j/kg.K and the specific heat capacity at constant volume is 741 j/kg.K. Please assume ambient pressure is zero absolute outside of the cylinder.
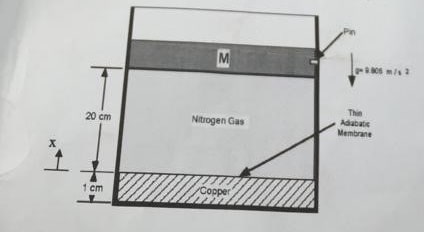
- What is the mass of the contained nitrogen gas?
- What is the mass of the copper?
The pin is removed and the piston settles to a new position (state2)
- What is the position of the piston in state 2?
- What are the temperature and volume of the nitrogen gas in state 2?
- What is the entropy change of the gas, piston and copper during the transition from state 1 to state 2?
The thin adiabatic membrane is now removed (with no work or heat transfer with the environment). After some time, the system settles in state 3.
- What is the temperature of the system in state 3?
- What is the position of the piston in state 3?
- What is the total entropy generated in the transition from state 2 to state 3? What is the sign of the entropy change for the copper block and nitrogen block?
Watch Solution on YouTube: https://www.youtube.com/watch?v=kRdMkZ7Robg
You are in charge of cooking 6 eggs for your friend. You can approximate an egg as being a sphere of diameter of 5cm. All eggs are initially in your fridge at a uniform temperature of 2oC. You lower the six eggs into a pot filled with boiled water at 100oC. You put the cover on the pot and removed the pot from the stove. You can assume that the cover pot is adiabatic (no heat transfer between the pot and the surrounding). After 15 minutes you removed the six egg from the pot and measure that each of them is at a uniform temperature of 80oC. The density and the specific heat of the water are ρw = 1000 kg/m3 and cw =4.187 kj/kg/k. The density and specific heat of an egg are ρe =1020kg/m3 and ce = 3.3kJ/kg/k. You can neglect the energy/release associated with the chemical and/or phase change that occur within each egg during cooking.
- Please describe a model suitable to determine the amount of heat transferred from water to heat egg?
- What is the magnitude of the energy lost by the water during 15 minutes cooking?
- If you measure the temperature of the water to be 95oC after 15 minutes of cooking. What was the volume of the water in the pot?
- Giving that volume of water obtained in (c) what is the maximum number of egg that you can cook to 70oC degrees for your friends
Consider Kaduna to Abuja train which as a normal operating speed of 80 KM/ hr. the train consist of a single locomotive and ten (10) passenger cars with a total mass of 4.4 X 105 Kg. it is been propelled by two engines capable of converting energy from fuel into mechanical power at the rate of 8.8MW. on a particular section of the track, the train enters a speed of 70km/hr and elevation of 0 meter and exist at a speed of 35km/hr at an elevation of 300m.
- Estimate the minimum work transfer required to accomplished this change of state. The minimum time required to carry out the process.
- If at the exist speed of 35km/hr , and elastic spring is now used to bring the train to rest in 0.1m,
- what will be the spring constant of this elastic spring?
Watch Solution on YouTube: https://www.youtube.com/watch?v=XxhjTiaIGQ8
Watch Solution on YouTube: https://www.youtube.com/watch?v=9jWOGZXwmP4
The adiabatic steady-flow device shown in the figure below has no shaft work transfer with the environment and the states of the gas at the two ports are as show.
- a) Please determine the mass flow rate per unit cross-section m1/A1 and the velocity of the fluid at sections 1 and 2
- b) Please determine whether the flow is from section 1 to section 2 or from section 2 to section 1. The gas can be modelled as an ideal gas with R=287 J/Kg.K and Cv = 716 J/Kg.K

Watch Solution on YouTube: https://youtube.com/watch?v=E66BVhshnqM
An adiabatic cylinder sketch below, contains nitrogen gas ( modeled as an ideal gas) and a block of copper separated by adiabatic membrane. Initially ( state 1), the pressure and temperature of the gas are 6 X 105 pa and 270K, respectively. The copper block temperature is 380 K. a heavy frictionless piston ( M = 45 kg is pinned at a height of 20 cm above the surface of the copper block. The copper block is 1 cm thick. The surface area of the piston is 50 cm2. The density of copper is 8.96 g/cm3. The specific heat capacity of copper is 385J/kg-k. the gas constant of nitrogen is 297 J/kg-k and its specific heat capacity at a constant volume is 741 J/kg-k. please assume ambient pressure is zero absolute outside of the cylinder
- a) What is the mass of the contained nitrogen gas?
- b) What is the mass of the copper?
The pin is removed and the piston settles to a new position ( state 2 )
- c) What is the position of the piston in state 2?
- d) What are the temperature and volume of the nitrogen gas in state 2?
Watch Solution on YouTube: https://youtube.com/watch?v=LpePvZOoOVA
As a part of a heat treating operation a steel part with a mass of 150kg is to be quenched from t temperature of 100 degrees to a temperature of 60 degree. Available as a quenching medium is a water bath at 20 degree enclosed in an adiabatic chamber. The specific heat of steel is 473j/kg-k and the specific heat of water is 4187j/kg-k
- Is there any heat transfer into ( or out of ) the system consisting of the steel part and the water?
- What is the minimum necessary amount of water to perform the quenching process ?
- If another liquid with a specific heat 10 times that of water was used for quenching the steel part, how much less/ more of this liquid would you need to complete the quenching.
Watch Solution on YouTube: https://youtube.com/watch?v=-lQ04R7J6J8
Pin Ball Game
Consider the game of pinball. In its typical form, the game consists of a steel ball of mass m = 67 grams that is lunched up a table inclined at an angle. The table has a length of L = 1.5 m and is fitted with a lunched track that is curved so that when the ball reaches the highest point of the track it has zero velocity. From this point, the ball starts to roll down the table under the influence of gravity striking various bumpers and flippers in its decent. The length is affected by having the operator compress a spring that is in contact with the ball at the rest position The spring is released and the ball begins its movement up the table, In this case, the spring is pure translational spring with spring constant k = 110 N/M and in its lunched position is compressed 6cm from free length position by the operator before being released. Our task is to estimate the value of.
- By taking only the ball as the system
- By taking the ball and the elastic spring as the system
Watch Solution on YouTube: https://youtu.be/RbKdbG0ZL-M
Consider an automobile travelling at a constant speed along a road. Determine the direction of the heat and the work interactions, taking the following as the system
- (a) the car radiator
- (b) the car engine
- (c) the car wheels
- (d) the road and
- (e) the air surrounding the car
Watch Solution on YouTube: https://youtu.be/O_k-crtzMS8
Do you have a Question to Ask?
SmartBukites have a Facebook Group which allows everyone to ask, answer and seek academic help. At SmartBukites, we believe Smart approach to Education will go along way in easing human academic struggle.
Click the link to join now!!! https://web.facebook.com/groups/675122219621351
